Part 5 of a 5-part blog series: “The Bradley Broadfield superphosphate process”
 The Broadfield process has remained a mainstay of the global phosphate industry since its introduction in the 1930s. In Part 1, Ian Hancock, Vice President Sales & Operations, Bradley Pulverizer, provides an introduction to the Bradley Superphosphate process, in Part 2 we explore the origins of superphosphate manufacturing , Part 3 outlines the chemical process for SSP manufacture, and Part 4 explains the overall system and processes. Click Here to download the complete article in pdf format as published by BCInsight Ltd in Fertilizer International Sept|Oct 2022, issue 510, pp. 31-35.
The Broadfield process has remained a mainstay of the global phosphate industry since its introduction in the 1930s. In Part 1, Ian Hancock, Vice President Sales & Operations, Bradley Pulverizer, provides an introduction to the Bradley Superphosphate process, in Part 2 we explore the origins of superphosphate manufacturing , Part 3 outlines the chemical process for SSP manufacture, and Part 4 explains the overall system and processes. Click Here to download the complete article in pdf format as published by BCInsight Ltd in Fertilizer International Sept|Oct 2022, issue 510, pp. 31-35.
 Acidulation in the Broadfield Den is a continuous process that requires good coordination between the various feed streams and equipment so they operate together in equilibrium. However, as ideal process conditions are almost never met in practice, Bradley’s Broadfield Dens and Belt Dens are designed to operate effectively at between 60-110 percent of their rated output. This provides operators with the necessary flexibility to adjust for changes in feed composition and/or plant conditions while ensuring that consistent end-product quality is maintained. Some of the key operational considerations are highlighted below.
Acidulation in the Broadfield Den is a continuous process that requires good coordination between the various feed streams and equipment so they operate together in equilibrium. However, as ideal process conditions are almost never met in practice, Bradley’s Broadfield Dens and Belt Dens are designed to operate effectively at between 60-110 percent of their rated output. This provides operators with the necessary flexibility to adjust for changes in feed composition and/or plant conditions while ensuring that consistent end-product quality is maintained. Some of the key operational considerations are highlighted below.
Rock blend. Individual phosphate rock sources and shipments will have different parameters for total phosphorus content, reactivity rates, impurity levels (i.e., cadmium), fluorine volatilization, and odorous compounds. To adjust for these differences, rocks from different sources are blended to establish the desired chemical composition and achieve the necessary ‘operational window’. This is important as the chemistry of the rock blend will directly influence the equilibrium of the entire system, including milling fineness, acid concentrations, mixer speeds, den residence time, and granulation conditions etc.
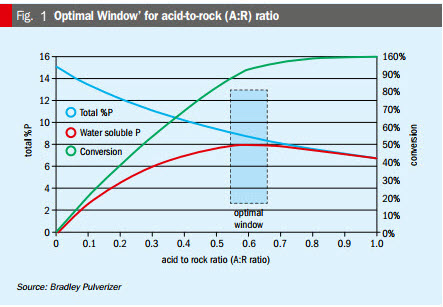
 Acid-to-rock ratio (A:R ratio). The phosphoric acid that is produced in the acidulation process initially increases the A:R ratio, even though it reacts subsequently to form calcium phosphate. This bump in acid generation must be properly accounted for when accurately determining the ideal A:R ratio (Figure 1). Additionally, high iron, aluminum, and magnesium levels must also be considered, as these will also reduce the achievable A:R ratio. These elements slow down the reaction rate by increasing the viscosity of the liquid phase and making the ex-den product sticky. These effects can be offset by increasing the fineness of the rock, increasing the residence time in the den, and increasing the reaction temperature.
Acid-to-rock ratio (A:R ratio). The phosphoric acid that is produced in the acidulation process initially increases the A:R ratio, even though it reacts subsequently to form calcium phosphate. This bump in acid generation must be properly accounted for when accurately determining the ideal A:R ratio (Figure 1). Additionally, high iron, aluminum, and magnesium levels must also be considered, as these will also reduce the achievable A:R ratio. These elements slow down the reaction rate by increasing the viscosity of the liquid phase and making the ex-den product sticky. These effects can be offset by increasing the fineness of the rock, increasing the residence time in the den, and increasing the reaction temperature.
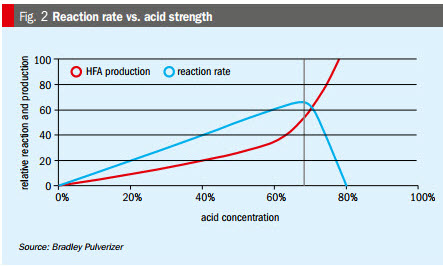
Acid strength. Optimal acid strength is around 68-70 percent (Figure 2), depending on the phosphate rock blend. Acid strength is an important parameter as it can negatively affect the reaction rate, as follows:
- If it is too weak, acid strength slows reaction rate by reducing reaction temperature and/or reducing reaction ‘chances’.
- If it is too strong, acid strength slows reaction rate by reducing liquid diffusion and particle-liquid contact, and/or blinding rock reaction surface with insoluble calcium sulphate. Strong acid also increases gaseous emissions.
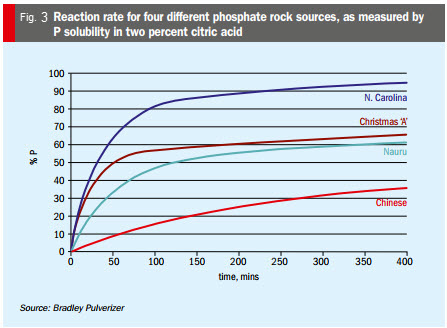 Reaction surface area. Reaction rate (as measured by two percent citric acid solubility, Figure 3) is affected by the available reaction surface area, which in turn is influenced by particle-size distribution, particle porosity, and crystal structure. Available surface reaction area is increased by milling fineness – although it is important to note that fineness:
Reaction surface area. Reaction rate (as measured by two percent citric acid solubility, Figure 3) is affected by the available reaction surface area, which in turn is influenced by particle-size distribution, particle porosity, and crystal structure. Available surface reaction area is increased by milling fineness – although it is important to note that fineness:
- Only affects rock reactivity
- Does not change overall conversion
- Has a strong effect on reactive rock, but a weak effect on non-reactive rock.
Summary
Large-scale commercial superphosphate production requires Broadfield process equipment that is rugged enough to:
- Continuously crush up to 50 t/h of phosphate rock
- Mix it with a steady stream of acid
- Enable the exothermic reaction required for proper acidulation.
Yet, this large-scale equipment must also be flexible enough to cope with, and adjust for, unavoidable changes to process variables – such as the natural disparities in phosphate rock chemistry. At any given time, the ongoing process reaction will be affected by:
- Reactivity of the rock and mill fineness
- Acid strength
- Reaction temperature
- Acid-to-rock ratio
- Mixing efficiency (mixer performance, time in mixer)
- Impurities, poisons and blinding effects.
In our experience, if just one of the above conditions is not correctly adjusted for, then acidulation will be sub-optimal.
Return to Part 1 – Introduction to the Bradley Broadfield Superphosphate Process
Back to Part 4 – Superphosphate Acidulation in the Broadfield Den
Contact Us to send us your rock samples for testing and we’ll design a Broadfield Process that meets the demands of your environment.
(US) 855-670-8777 | (International) 44-0808-196-8141
